WHAT IS ISO 14801? WHY IS IT CRITICAL?
The ISO 14801 standard specifies a method for testing dental implants with their premanufactured prosthetic components. It is designed to simulate the “worst-case scenario” of functional loading in the oral cavity to compare different implant designs and sizes.
- Scope: Single post endosseous dental implants and their prosthetic parts.
- Exclusions: Implants shorter than 8mm or those utilizing magnetic attachments.
- Objective: To determine the fatigue limit and the S-N curve (Wöhler curve) through cyclic loading.
ISO 14801 GENERAL PRINCIPLES AND TEST REQUIREMENTS
To ensure valid results, the test must replicate clinical conditions as closely as possible:
- Specimen integrity: Specimens must represent the final product, including identical manufacturing and sterilization processes.
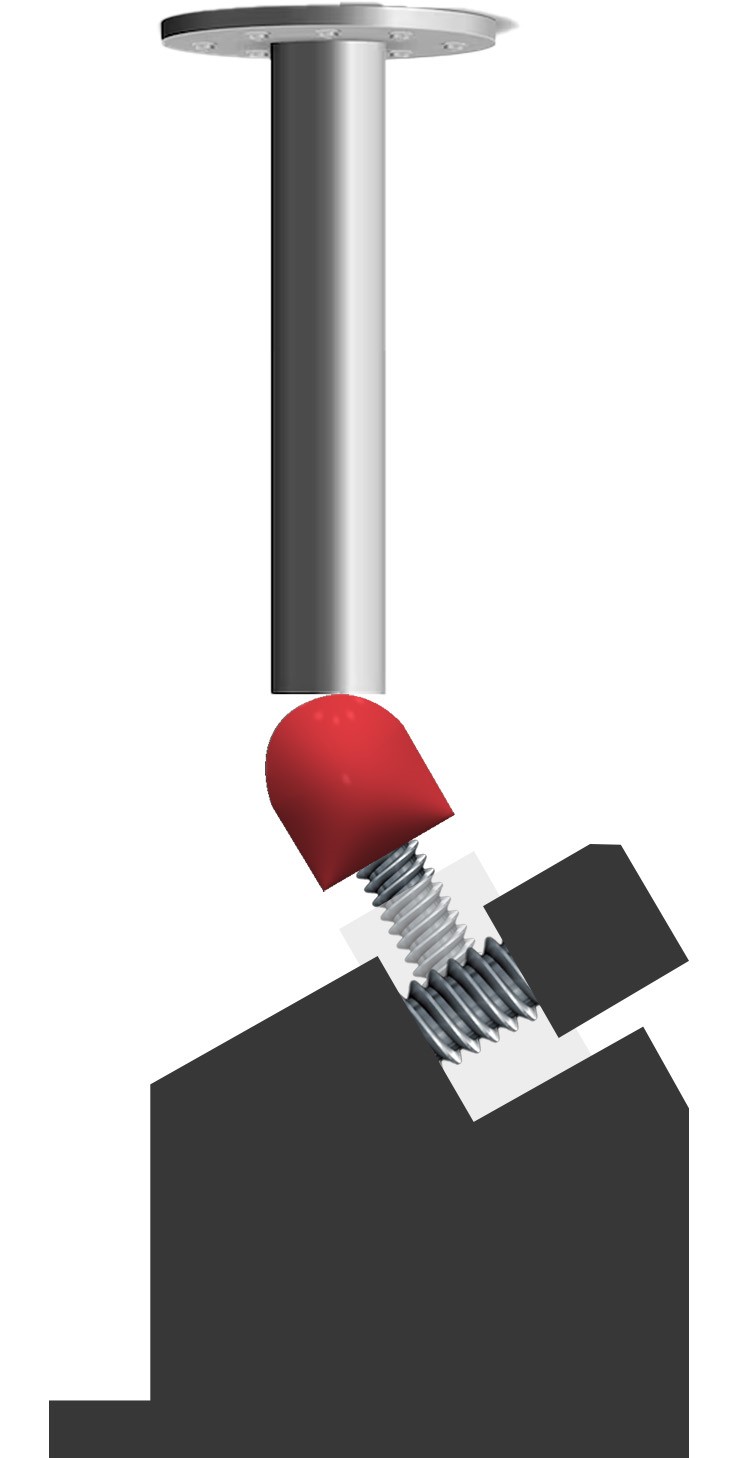
- Worst-Case configuration: Testing must be performed on the configuration most susceptible to failure (e.g., longest abutment or smallest diameter), as detailed in ISO 14801 Annex B.
- Assembly precision: Screw joints must be tightened within ±5% of the manufacturer’s recommended torque.
- Loading conditions: The test applies a sinusoidal peak load at a specific angle (typically 30°) to simulate the vectors of human chewing forces.
TECHNICAL SPECIFICATIONS FOR THE TEST SYSTEM
According to ISO 7500-1, the testing machine must guarantee extreme precision. A compliant system must include:
| Feature | ISO 14801 Requirement | STEP Lab EA Series Advantage |
|---|---|---|
| Load accuracy | Within ±5% of maximum load (ISO 7500-1) | High-precision load cells with 0.5 class accuracy |
| Frequency | Up to 15 Hz (in air) or 2 Hz (in saline) | Fully adjustable dynamic frequency control |
| Cycle count | 2 to 5 million cycles | Automated stop and failure detection system |
| Environment | Optional saline/physiological bath | Integrated temperature-controlled fluid cells |
TESTING MACHINES FOR ISO 14801
Our testing machine from EA Series is our premier solution for dental implantology.
EA systems offer:
- Superior accuracy in both force and position control for generating precise S-N curves.
- Zero maintenance and low energy consumption, ideal for long-duration fatigue tests (5+ million cycles).
- Capable of dynamic loads up to 200kN, covering everything from single implants to multi-unit structures.
- “Plug & Play” installation with an intuitive interface.
MAXIMIZE PRODUCTIVITY: THE NEW 5-AXIS MULTI-STATION SYSTEM
STEP Lab introduces the 5-Axis Dynamic Testing Machine, specifically engineered to scale up your ISO 14801:2016 compliance workflow. This table-top powerhouse integrates five independent testing stations based on our newest electromechanical EA02 actuators, allowing you to characterize an entire batch of implants simultaneously, drastically reducing time-to-market.
Unmatched efficiency
Powered by five state-of-the-art EA02 electromechanical actuators, ensuring clean, quiet, and maintenance-free operation.
- Max. Displacement: 150 mm (±75 mm)
- Max. Dynamic load: 1300 N
- Max. Static load: 800 N
- Max. Speed: 150 mm/s
Precision at scale
Each station features an independent Class 0.5 ISO 7500-1 load cell, guaranteeing that every single test meets the highest accuracy standards for dynamic loads.
Total versatility
Beyond the standard 30° ER collet fixtures, the system is fully customizable. Perform static (creep, relaxation) and dynamic (fatigue) tests using our Test Center software, which allows for point-by-point profile definition.
In-Vivo simulation
Each station can be equipped with an independent container for saline media testing, temperature-controlled up to 40°C to replicate the human oral environment perfectly.