Testing machines play a crucial role in ensuring the quality and safety of medical devices and components. Among the essential standards for this evaluation is ASTM F1717, which provides guidelines for both static and fatigue testing of spinal implants. Adherence to ASTM F1717 ensures that spinal implants meet rigorous mechanical requirements, contributing to their effectiveness and reliability in clinical use.
ASTM F1717: WHAT IS?
ASTM F1717 comprises a set of testing standards developed to define specific methods for testing spinal implants both statically and under fatigue conditions, within a vertebrectomy model context. These standards are essential for evaluating the mechanical performance of spinal implants, enabling comparison between different configurations and applications, as well as different designs. Essentially, they provide guidelines for assessing the performance of spinal implants in various anatomical contexts and with different methods of application to the spine.
ASTM F1717: WHAT DOES IT MEASURE?
ASTM F1717 standards focus on evaluating the static and dynamic mechanical characteristics of spinal implants. The associated mechanical tests are conducted in vitro, using simplified loading schemes that, although unable to accurately replicate the complex loads acting on the spine in vivo, still allow for the comparison of different spinal implant configurations in terms of relevant mechanical parameters.
ASTM F1717: TEST CONDITIONS
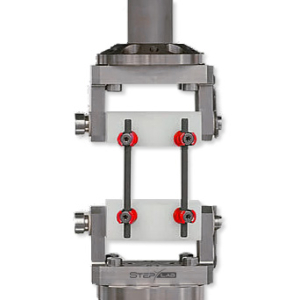
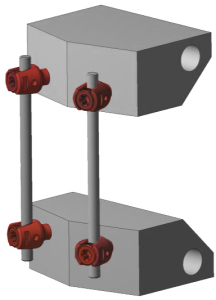
In general, six specimens are tested, with two UHMWPE blocks used for implant fixation. The dynamic loading is conducted at a frequency of 5 Hz and up to 5 million cycles. Regular tests are performed in air at room temperature. However, if the implant material requires test setups simulating body environmental conditions at 37°C in saline solution, these conditions are accommodated.
The standard outlines four test procedures: static and dynamic axial compression bending, static tension bending, and static torsion. It is applicable to both lumbar and cervical systems.
ASTM F1717: TYPES OF TEST
ASTM F1717 standards outline four main types of testing:
Compression Bending Test
- Test Purpose: This test evaluates the strength and stability of spinal implants when subjected to compressive and flexural loads similar to those experienced in the human spine.
- Test Procedure:
- Sample Preparation: Ultra-High-Molecular-Weight Polyethylene (UHMWPE) blocks are selected to simulate vertebral bodies. Anchors required for sample installation are prepared according to the manufacturer’s instructions.
- Spinal Implant Mounting: The spinal implant is assembled and installed between the UHMWPE blocks, adhering to the manufacturer’s specifications.
- Load Application: The load is applied at the speed specified in ASTM F1717 standards (usually 25 mm/min) until reaching the yield point or beyond.
- Evaluated Parameters: Load-displacement curves are recorded during the test to assess the stiffness, yield load, and ultimate load of the spinal implant.
- Displacement at 2% offset yield (mm)
- Elastic displacement (mm)
- Compressive bending yield load (N)
- Compressive bending stiffness (N/mm)
- Compressive bending ultimate displacement (mm)
- Compressive bending ultimate load (N)
Tension Bending Test
- Test Purpose: This test evaluates the strength of spinal implants when subjected to tensile and flexural loads, simulating real-world usage conditions.
- Test Procedure:
- Sample Preparation: The same sample preparation procedures are followed as in the compression bending test.
- Spinal Implant Mounting: The implant is installed between the UHMWPE blocks.
- Load Application: The load is applied according to the established protocol, with recording of load-displacement curves and evaluation of strength parameters.
- Data Analysis: Evaluate parameters, including:
- Displacement at 2% offset yield (mm)
- Elastic displacement (mm)
- Tensile bending yield load (N)
- Tensile bending stiffness (N/mm)
- Tensile bending ultimate displacement (mm)
- Tensile bending ultimate load (N)
Static Torsion Test
- Test Purpose: This test evaluates the ability of spinal implants to withstand torsional forces, similar to those experienced during rotational movements of the spine.
- Test Procedure:
- Sample Preparation: Test samples are prepared following the instructions specified in ASTM F1717 standards.
- Spinal Implant Mounting: The implant is installed between the UHMWPE blocks.
- Application of Torsional Moment: A specified torsional moment is applied to the ends of the implant, while data on deformation and resistance are recorded.
- Data Collection: Record torque-angular displacement curves and determine:
- Angular displacement at 2% offset yield (degrees)
- Elastic angular displacement (degrees)
- Yield torque (N-m)
- Torsional stiffness (N-m/degree)
Compression Bending Fatigue Test
- Test Purpose: This test evaluates the resistance of spinal implants to cyclic loads, similar to those experienced during daily activities.
- Test Procedure:
- Sample Preparation: Test samples are prepared following the instructions specified in ASTM F1717 standards.
- Spinal Implant Mounting: The implant is installed between the UHMWPE blocks.
- Load Cycles: A repeated cyclic load is applied at a specified frequency for a predetermined number of cycles, while monitoring the deformation and resistance of the implant.
- Evaluation: Monitor initial and secondary failures, record failure modes, and assess deformations. Determine the endurance limit and plot a semi-log fatigue curve of compression bending load versus the number of cycles at failure.
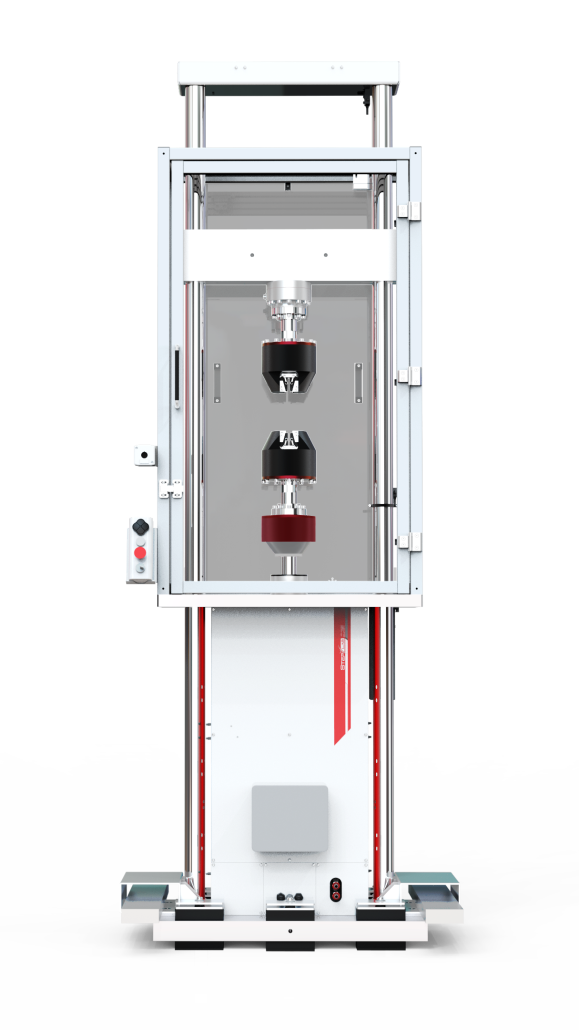
TESTING MACHINES FOR ASTM F1717
At STEP Lab, we offer multi-actuator systems for tensile and torsion testing. BV systems are available in a wide range of loads, with torque capacities up to 230 Nm. The torsional motor is coupled to every linear actuator series we manufacture, from electromechanical actuators, EA series and all UD, HUD and XUD series based on linear motors.
- Control of two or more synchronised axes
- No maintenance
- High efficiency
- Easy installation